Immunotherapy with Chimeric Antigen Receptor (CAR) T cells against CD19 can cure patients with refractory B cell lymphoma and leukemia. However, complications of CAR-T cell therapy can cause morbidity. Among the complications of CAR-T cell therapy is cytopenia, i.e. decreased peripheral blood counts of platelets, leukocytes and / or erythrocytes. The exact mechanism of this side - effect is not known, retrospective analysis showed it affects 10-20% of patients and is linked with the severity of cytokine release syndrome (Juluri et al, PMID34666344). We have previously developed a mouse model of CAR-T cell therapy using C56BL/6 mouse expressing human CD19 under a B- Cell specific promoter (Pennell et al, PMID29735365). The recipient mice are heterozygous for human hCD19 on CD45.2 background. Congenic C56BL/6 CD45.1 mice served as T cell donors. The aim of this study was to investigate if the mouse model recapitulates cytopenia seen in patients after CAR-T cell therapy.
C56BL/6 recipient mice were treated with lymphodepleting chemotherapy (Cyclophosphamide (CY) 300 mg/kg) and infused with 1) no T cells 2) control T cells transfected with hEGFR 3) T cells transfected with CD19 CAR with hCD137 and hCD3zeta signaling domains. The chosen dose of infused T cells at 0.3E+6 per animal was low, approximately 10x below the lethal dose and 3x less than IC50 dose, to reduce early mortality. The animals were weighed and examined regularly, and appearance and activity were scored from 0 (healthy) to 8 (moribund). Total of 30 animals per group were harvested at 5 time points, 6 mice each, on days 5, 12, 26, 40 and 54. Peripheral blood counts were measured using HemaVet CBC counter. Flow cytometry was performed on blood and bone marrow. Spleens, long bones (femur, tibia) and sternum were harvested for histopathologic assessment. Multiparametric immunohistochemistry (IHC) was performed using Codex (Phenocycler, Akoya).
The mice receiving CAR-19 T cells showed significant, but transient loss of weight and transient increase in morbidity score during the first 2 weeks. Mortality was not significantly different between groups: 0, 1 and 2 deceased animals in CY, control T and CAR-T cell groups, respectively. We observed transient significant cytopenia in the CAR-T cell treated mice, with significantly lower platelet counts on day 5 (p<0.05) and lower White Blood Cell counts on days 12 (p<0.06) and day 26 (p<0.05).
These changes were associated with 100-fold larger expansion of CAR-T cells in blood as compared to the control hEGFR T cells and 20-fold higher relative expansion of CAR-T cells in the bone marrow.
Multiparametric IHC showed loss of B cell follicles and increased density of donor T cells in the CAR-T cells recipients compared to hEGFR T cell control.
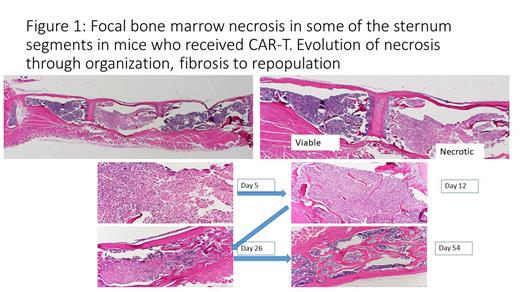
Pathologic evaluation of sternums revealed individual segments (sternebra) with necrosis of the hematopoietic elements at the first time point (day 5). At subsequent time points there were progressive changes reminiscent of evolution of infarct with organization, proliferation of fibroblasts and fibrosis with increased osteogenesis and finally with regression of mesenchymal elements and reestablishment of hematopoiesis (Figure 1). The number of segments with necrosis and sequalae was significantly higher in the CAR-T cell group as compared to the hEGFR T cell control (p=0.0005). The necrosis of hematopoietic elements within individual sternum segments involved at the most 3 segments out of 6. The involved segments appeared to distribute randomly: either adjacent or separated by viable segments. The necrotic segments showed paucity of erythrocytes with foci of recent erythrocyte extravasation consistent with ischemic infarct.
The mouse model of CAR-T cell therapy against CD19 recapitulates cytopenia in early post - treatment period. The observed pattern of necrosis of the hematopoietic elements in entire segments suggests compromised blood supply. We hypothesize sternebra are more susceptible to necrosis due to limited blood supply, separate for each segment. We hypothesize that occlusion of arteries or capillaries in the bone marrow may contribute to the mechanism of cytopenia following CAR-T cell therapy. Prior retrospective analysis linked the cytopenia with increased levels of inflammatory mediators of Cytokine Release Syndrome. The present findings highlight a role of local factors in situ, which may be influenced by systemic cytokines.
Disclosures
Blazar:BlueRock Therapeutics: Current Employment, Membership on an entity's Board of Directors or advisory committees; Carisma Therapeutics: Current Employment, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal